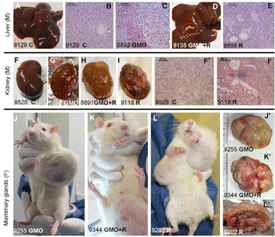
The editor of the journal Food and Chemical Toxicology (FCT), Dr A. Wallace Hayes, has decided to retract the study by the team of Prof Gilles-Eric Séralini, which found that rats fed a Monsanto genetically modified (GM) maize NK603 and tiny amounts of the Roundup herbicide it is grown with suffered severe toxic effects, including kidney and liver damage and increased rates of tumours and mortality.[1] GMWatch believes FCT’s retraction of Prof Séralini’s paper to be illicit, unscientific, and unethical. It violates the guidelines for retractions in scientific publishing set out by the Committee on Publication Ethics (COPE),[2] of which FCT is a member.[3]. Video: http://www.activistpost.com/2013/12/how-monsanto-silences-scientific-di…
COPE guidelines state that the only grounds for a journal to retract a paper are:
•Clear evidence that the findings are unreliable due to misconduct (eg data fabrication) or honest error
•Plagiarism or redundant publication
•Unethical research.
Prof Séralini’s paper does not meet any of these criteria and Hayes admits as much. In his letter informing Prof Séralini of his decision (available at: http://www.gmwatch.org/index.php/news/archive/2013/15184), Hayes concedes that an examination of Prof Séralini’s raw data showed “no evidence of fraud or intentional misrepresentation of the data” and nothing “incorrect” about the data.
Hayes states that the retraction is solely based on the “inconclusive” nature of the findings on tumours and mortality, given the relatively low number of rats used and the choice of rat strain, which Hayes says naturally has a “high incidence of tumours”.
Crucially, however, inconclusiveness of findings is not a valid ground for retraction. Numerous published scientific papers contain inconclusive findings, which are often mixed in with findings that can be presented with more certainty. It is for future researchers to build on the findings and refine scientific understanding of any uncertainties.
It is important that scientists do not overstate their findings or draw conclusions that are not justified by the data, but Prof Séralini’s paper does not do this. Because Prof Séralini’s study was a chronic toxicity study and not a full-scale carcinogenicity study, which normally requires larger numbers of rats, he conservatively did not do a statistical analysis of the tumours and mortality findings. Instead he simply reported them, without drawing definitive conclusions. This is in line with the OECD chronic toxicity protocol, which requires that any “lesions” (including tumours) observed are recorded.[4]
The criticisms of the low number of rats and choice of rat strain have been addressed by Prof Séralini’s team in a comprehensive response to critics that was published in FCT,[5] as well as by independent scientists writing in support of the study.[6]
Experts in statistics writing in support of the study have pointed out that large numbers of animals are only required in safety studies to avoid false negative error, where a toxic effect exists but is missed because too few animals are used. In the case of Séralini’s study, this was not an issue. The toxic effects of the test substances were so pronounced (there was a “large effect size”) that smaller numbers of animals were sufficient for statistical significance.[7,8,9]
Regarding the Sprague-Dawley strain of rat that was used, all strains of rodents develop spontaneous tumours with age, as do humans. The fact that there is a low level of spontaneous tumour occurrence in the control group in Séralini’s study mimics the human condition. For this and other reasons, most toxicology studies use this strain of rat.
Hayes fails to address these responses and arguments in support of the study, raising questions about the expertise, balance, and objectivity of his anonymous review panel. In addition, the legitimate peer reviewers had previously considered these aspects of Séralini’s study and nevertheless decided that “the work still had merit” and should be published.
In a highly irregular process, Hayes now contradicts the outcome of the peer review and editorial process and decides to retract the paper over a year after it was published. His decision is not made on the basis of new data, but on a secret and non-transparent review by unnamed persons, who evidently do not feel able to stand behind their decision publicly or disclose any conflicts of interest they may have.
Hayes’ decision will tarnish the reputation of FCT and will increase public mistrust of science in general and genetically modified foods in particular.
The Goodman factor
Hayes’ decision to retract the paper follows FCT’s appointment of Richard E. Goodman, a former Monsanto scientist and an affiliate of the GMO industry-funded group, the International Life Sciences Institute, to the specially created post of associate editor for biotechnology at the journal, early this year.[10]
Goodman’s appointment in turn followed an orchestrated campaign by GMO supporters to persuade FCT to retract the study. Some critics even accused Prof Séralini of fraud, without presenting any evidence. Many of the critics had undeclared conflicts of interest with the GMO industry.[11]
After Goodman was installed, FCT withdrew a separate study by Brazilian researchers that also raised questions about GM crop safety. The study showed that Bt insecticidal toxins similar to those engineered into GM Bt crops were not broken down in digestion, as is claimed by the industry and regulators, but had toxic effects on the blood of mice. The Brazilian paper, like Prof Séralini’s, had been peer-reviewed and published by FCT prior to Goodman’s arrival. After Goodman’s arrival, the paper was withdrawn without explanation from FCT[12] – only to be immediately published in another journal.[13]
There is no proof that Goodman was responsible for the retraction of Prof Séralini’s study. But his appointment, coming so soon after the “Séralini affair”, along with FCT’s failure to list the interests of its editors, raises questions about corporate influence on the editorial board at the journal.
Notes
1. Séralini GE et al (2012) Long term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize. Food and Chemical Toxicology, 50(11): 4221-4231.
2. http://publicationethics.org/files/retraction%20guidelines.pdf
3. http://publicationethics.org/members/food-and-chemical-toxicology
4. Organisation for Economic Cooperation and Development (OECD) (2009). OECD guideline no. 452 for the testing of chemicals: Chronic toxicity studies: Adopted 7 September 2009. http://bit.ly/LxJT1Z
5. Séralini GE et al (2013). Answers to critics: Why there is a long term toxicity due to NK603 Roundup-tolerant genetically modified maize and to a Roundup herbicide. Food and Chemical Toxicology 53: 461-468. http://www.ncbi.nlm.nih.gov/pubmed/23146697
6. http://gmoSéralini.org/faq-items/what-was-the-reaction-to-the-study-2/
7. Deheuvels P. Étude de Séralini sur les OGM: Pourquoi sa méthodologie est statistiquement bonne [Seralini study on GMOs: Why the methodology is statistically sound]. Le Nouvel Observateur. 9 October 2012. http://bit.ly/RtPivG
8. Saunders P. Excess cancers and deaths with GM feed: The stats stand up. Science in Society. 16 October 2012. http://www.i-sis.org.uk/Excess_cancers_and_deaths_from_GM_feed_stats_st…
9. Deheuvels P. L’étude de Séralini sur les OGM, pomme de discorde à l’Académie des sciences [The Seralini GMO study - A bone of contention at the Academy of Sciences]. Le Nouvel Observateur. 19 October 2012. http://leplus.nouvelobs.com/contribution/661194-l-etude-de-seralini-sur…
10. http://www.independentsciencenews.org/science-media/the-goodman-affair-…
11. http://www.spinwatch.org/index.php/issues/science/item/164-smelling-a-c…
12. Mezzomo BP et al (2012). WITHDRAWN: Effects of oral administration of Bacillus thuringiensis as spore-crystal strains Cry1Aa, Cry1Ab, Cry1Ac or Cry2Aa on hematologic and genotoxic endpoints of Swiss albino mice. Food Chem Toxicol. http://www.ncbi.nlm.nih.gov/pubmed/23146696
13. Mezzomo BP et al. (2013). Hematotoxicity of Bacillus thuringiensis as spore-crystal strains Cry1Aa, Cry1Ab, Cry1Ac or Cry2Aa in Swiss albino mice. J Hematol Thromb Dis 1(1).
Source:
Claire Robinson (Managing Editor of GMO Seralini), 28 November 2013
http://gmoseralini.org/journal-retraction-of-seralini-study-is-illicit-…
- Log in to post comments

New EFSA Guidance validates Seralini's study
The European Food Safety Authority (EFSA) has issued guidelines for two-year whole food feeding studies to assess the risk of long-term toxicity from GM foods.
http://www.efsa.europa.eu/en/efsajournal/pub/3347.htm
This is a fascinating document which largely validates the methodology and choices of Prof GE Seralini in his 2012 study on GM maize NK603 - methodology and choices that EFSA and countless other critics previously attacked him for.
Particular points to note:
1. EFSA admits that "no standardised protocol or guidelines exist for this type of study and [industry] applicants have to adapt protocols" - as Seralini did, too.
2. EFSA says the same strain of rat that was used in the 90-day study on the GM food should be used in the longer study - thus vindicating Seralini's use of the Sprague-Dawley rat, which Monsanto used in its 90-day study on the same maize.
3. EFSA says animals should be fed ad libitum, which Seralini did, but which critics complained made it impossible to measure individual food and water consumption.
4. EFSA admits that you do not necessarily need a narrow and fixed hypothesis and that such a study can be "exploratory", in spite of its previous claim that Seralini's experiment was flawed because it (according to EFSA) didn't have a clear hypothesis or objective.
5. EFSA recommends against using the extra control or "reference diet" groups commonly included by Monsanto in its 90-day studies and fed a variety of supposedly non-GM diets, on the grounds that the concurrent controls are the valid controls AND what is being tested is the difference between the GM variety and the non-GM comparator. Seralini was criticised by many for not including these spurious extra control groups and for thus having "inadequate controls".
6. EFSA cautions strongly AGAINST relying on historical control data and if it is used, restricts it to within 5 years of the current experiment and to the same testing facility. This is a much stricter requirement than industry ever applies; industry uses ancient data from a wide variety of sources.
EFSA says: "The use of historical control data should be considered with caution. The historical controls might not be useful because the incidences of neoplastic (or non-neoplastic) lesions would possibly be from control animals kept on different diets than the diet applied in whole food/feed study, and because the diet itself (high/low fat, type of fat, % of carbohydrate, type of carbohydrate, etc.) can influence the formation of neoplastic or non-neoplastic lesions. Where the diet formulation used in the experiment for the control groups cannot be demonstrated to be equivalent to that used for the generation of historical control data, the inclusion may be considered of an additional control group (as similar as possible to the historical controls), in addition to the concurrent control group(s)."
It's unfortunate that in rightly condemning the use of historical control data, however, EFSA allows in those extra control or "reference" groups that it rightly condemned in point (5) above.
7. EFSA recommends a minimum of 10 animals per sex per group for the chronic toxicity phase, the same number that Seralini used.
8. EFSA recommends housing animals in pairs, as Seralini did, so individual food consumption cannot be measured.
9. EFSA requires an a priori power analysis to ensure appropriate sample size, depending on the effect size that is being looked for. We've never noticed the GM industry doing one of these, resulting in experiments that are virtually guaranteed not to find anything. For Seralini's team's comment on this, see:
http://www.ijbs.com/v05p0706.htm
Overall, we're pleased to see EFSA taking on board our cautionary lessons on spurious "reference" control groups and historical control data (even if in the same document EFSA subsequently allows the use of both!), as well as validating the aspects of Seralini's experiment that he was most criticised for.
Source: Claire Robinson of GMWatch and Earth Open Source, 31 July 2013
http://www.gmwatch.org/index.php/news/archive/2013/14882-seralini-valid…
Séralini is now threatening to sue Food & Chemical Toxicology
In 2012, Gilles-Eric Séralini of the University of Caen, France, and his colleagues published results in Food and Chemical Toxicology showing that genetically modified (GM) maize caused tumors in rodents.
Last month, the editors of the journal decided to retract the article after having reviewed the raw data and determined that the results were inconclusive. Now, Séralini is threatening to sue the journal and nearly 200 scientists have signed a petition to boycott its publisher, Elsevier.
“The article was explosive,” according to a write-up in The Economist. It fanned the flames of the anti-GMO movement and “had all the more impact because it contradicted previous studies on GM foods.” Yet it had some problems, according to the editors of Food and Chemical Toxicology. After a post-publication review, the editors determined that the number of animals used in the study was too low and called into question the paper’s conclusions. “Ultimately, the results presented (while not incorrect) are inconclusive, and therefore do not reach the threshold of publication for Food and Chemical Toxicology,” according to a statement from the journal.
“This arbitrary, groundless retraction of a published, thoroughly peer-reviewed paper is without precedent in the history of scientific publishing, and raises grave concerns over the integrity and impartiality of science,” nearly 200 signatories wrote in a petition to Elsevier. The group said it will no longer publish, purchase, or review articles in Elsevier publications unless the retraction is reversed.
According to Forbes, Séralini is now threatening to sue the journal. The researcher and his colleagues wrote on gmoseralini.org that they “do not accept as scientifically sound the debate on the fact that these papers are inconclusive because of the rat strain or the number of rats used. We maintain our conclusions.”
Source: The Scientist, 5 December 2013
http://www.the-scientist.com/?articles.view/articleNo/38566/title/GMO-R…